BioPharma
-
HHS Will Likely Face More Lawsuits Against Drug Price Negotiation Now That It Has Named the First 10 Meds Included In the Plan
Novartis became the seventh drugmaker to legally challenge the White House’s Medicare drug pricing negotiation program. The company filed its lawsuit three days after HHS announced the first 10 drugs that were selected for the program — its heart failure medication Entresto was one on the list. HHS can expect to face more lawsuits from the manufacturers of the 10 drugs it named last week, experts have warned.
-
Star Therapeutics Lands $90M to Expand Its Drug Discovery Universe
Startup Star Therapeutics will use the Series B funding to support clinical development of a drug candidate for von Willebrand disease, a bleeding disorder. The capital will also support the formation of new biotech subsidiaries developing antibodies with therapeutic applications in hematology and immunology.
-
Applying Remote Patient Monitoring to Surgery Prep and Recovery, Oncology and Women’s Health
Join us to learn about the latest trends in remote monitoring and how to extend its benefits beyond chronic conditions to more patients – all while using fewer staff resources.
-
Twice Rejected Diabetes Drug/Device Product Gets New Chance at Startup i2o
Intarcia Therapeutics’ GLP-1 agonist product candidate for type 2 diabetes now belongs to i2o Therapeutics, which will make the treatment’s case for approval at an upcoming FDA advisory committee meeting. The startup’s $46 million Series A financing is one of several recently announced funding rounds.
-
Amgen, FTC Settlement Allows $28B Horizon Acquisition to Move Forward
Amgen and the Federal Trade Commission are settling the lawsuit the regulator filed to block the pharmaceutical giant’s $28 billion Horizon Therapeutics acquisition. As part of the settlement, Amgen agrees not to “bundle” its products with Horizon’s drugs in negotiations with health plans.
-
Senate Bill Proposes Slashing ‘Red Tape’ Around Biosimilar Interchangeability
Legislation introduced by Utah Senator Mike Lee would eliminate testing to show a biosimilar can be substituted for a reference biologic product. Lee contends the current testing requirement adds costs and delays market access for these lower-cost biological medicines.
-
MedCity Influencers, BioPharma
The Inflation Reduction Act’s Impact on Drug Development for Rare Diseases
Despite these wins for many patients, the new law is already impacting the discovery and development of new drugs for people living with orphan diseases. Not only are drugs that could treat more than one disease being disincentivized, small molecule medicines, which play an important role in treating neurological disorders, cancers, and other diseases, may also be disadvantaged by the law.
-
Apellis Lays Off 25% of Staff and Turns Focus to Commercializing New Eye Drug
The Apellis Pharmaceuticals restructuring will save up to $300 million. It comes as the biotech continues its inquiry into the cause of a rare inflammatory complication reported in a small number of patients who received its geographic atrophy drug, Syfovre.
-
HHS Drops First 10 Drugs for Medicare Drug Price Negotiation
HHS released the first 10 drugs selected for the Medicare Drug Price Negotiation Program. These drugs include Eliquis, Jardiance and Xarelto. Under the program, the federal government will be able to negotiate the price for selected drugs for the first time.
-
Discover the Next-Gen Platform for Integrated Collaborative Care
Beyond EHRs and digital front doors, reducing the gaps in patient care journeys.
-
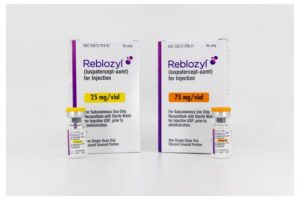
BMS Cancer Drug’s New FDA Nod Puts It on Path to Reach Blockbuster Status
Bristol Myers Squibb drug Reblozyl is now approved as a first-line therapy for anemia caused by myelodysplastic syndromes, a group of blood cancers. It’s the third indication for the drug, which analysts project could top $4 billion in peak sales.
-
Bayer Pushes Ahead With Parkinson’s Cell Therapy on Heels of Positive Phase 1 Data
A Bayer cell therapy for Parkinson’s disease has met primary and secondary goals of its first test in humans, and the pharmaceutical giant is now planning for a larger Phase 2 clinical trial expected to begin enrollment in the first half of next year.
-
Danaher Continues M&A Streak, Buying Life Sciences Firm Abcam for $5.7B
Abcam’s acquisition comes two months after its board announced the exploration of strategic alternatives that could include the sale of the company. Danaher beat out at least 20 companies that were potential buyers of the life sciences tools and antibody development firm.
-
The Journey to Personalized Healthcare
The Walt Disney Company excels in designing exceptional service because they are hyper-focused on listening, tracking, designing, and measuring the experiences of guests visiting their properties. We can apply this approach in pharma by enhancing our patient journey maps and go beyond focusing on the key clinical engagement touch points (e.g., doctor visits, prescription pickups) and enriching our insights with the micro-moments surrounding these obvious events.
-
New MD Anderson Research Uncovers Drug Combo That Could Eliminate Pancreatic Cancer Tumors
Researchers at the University of Texas MD Anderson Cancer Center published two studies this week on a new approach that could improve treatment for patients with pancreatic cancer. The preclinical studies showed that combining immunotherapy with a KRAS inhibitor can lead to long-lasting tumor elimination.
-
Skin Microbiome and Allergies: Understanding the Role of Microbes in Allergic Skin Conditions
Future research should focus on identifying the interactions between the immune system, microbes, and allergic responses; and exploring potential therapeutic strategies targeting the skin microbiome for allergy management.