

Name of Company: Ceribell Inc
Rafael Donnay, vice president of product and reimbursement at the Sunnyvale, California, company, describes the details of the FDA clearance of the ClarityPro software system.
Name of the Product:
ClarityPro
When did you receive the clearance?:
May 23, 2023
Describe the problem that the product addresses:
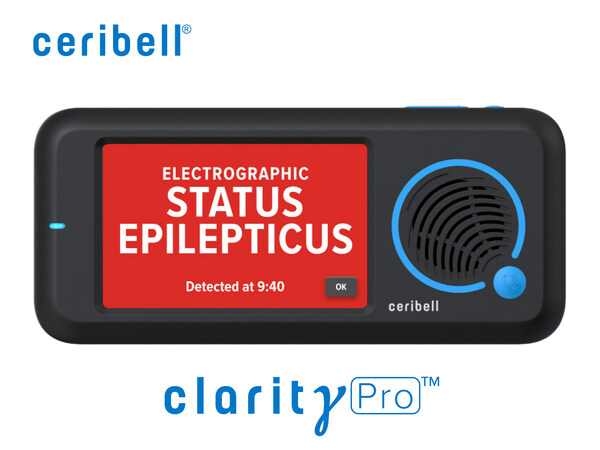
Diagnosis of ESE (Electrographic Status Epilepticus) is essential to treating prolonged seizures, which may affect one-third of hospitalized neurological critical care patients. These seizures increase the risk of permanent brain injury. Early diagnosis & management can dramatically improve patient outcomes. ClarityPro is now the first and only device to diagnose ESE according to the American Clinical Neurophysiology Society’s definition of ESE.
Who is the end-user?:
Healthcare providers in ICUs and Emergency Departments
Does the product need reimbursement and is it covered?:
Additional Medicare reimbursement will expand access to this vital technology to more Americans, especially seniors and critically ill patients who are more likely to suffer from neurological illnesses. With this approval, hospitals can receive NTAP reimbursement of up to $913.90 for eligible Medicare patients starting in October 2023.
What are the competitive products on the market?:
Zeto Wireless EEG Headset
Photo: jayk7, Getty Images and Ceribell














