FDA Clearance
-
Devices & Diagnostics, Health Tech
FDA Clears New Surgical Platform Combining Robotics & Magnetics
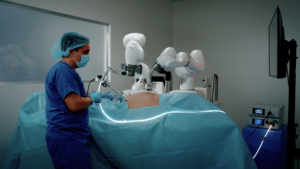
The FDA cleared Levita Magnetics’ new abdominal surgery platform, which combines the company’s proprietary magnetic surgical technology with robotic assistance. The system is designed to deliver all the same patient benefits as its original magnetic surgery system — such as reduced incisions, less pain, fewer scars and speedier recovery — while also giving surgeons better control of their instruments.
-
Devices & Diagnostics, Artificial Intelligence
FDA Clearance Spotlight: UltraSight
UltraSight, based in Israel, recently received a nod from the FDA to market its UltraSight AI Guidance product that can assist medical professionals without sonography experience in acquiring cardiac ultrasound images and allow for more widespread detection of heart diseases.
-
Payer’s Place: Dawn Maroney
Dawn Maroney, President, Markets of Alignment Health and CEO of Alignment Health Plan, to discuss how they are using technology to provide better service and care to consumers.
-
FDA Clears TytoCare’s New Algorithm for Wheeze Detection
Virtual care company TytoCare has received FDA clearance for its wheeze detection algorithm, allowing the company to begin commercializing the product in the U.S. The new algorithm is an expansion of Tyto Insights, the company’s AI-powered diagnosis support software.
-
In a first, FDA clears video game for kids with ADHD
Digital health company Akili Interactive received FDA de novo clearance for its digital therapeutic, a video game intended to improve attention among kids with ADHD.
-
Devices & Diagnostics, Health Tech
With $55M in new funding, Bigfoot Biomedical takes strides toward FDA submission
Bigfoot Biomedical plans to submit an application to the FDA for clearance of its smart insulin pen caps this month. The devices pull in information from a connected glucose monitor to help users calculate the optimal insulin with each meal.
-
FDA considering imposing 10-year limit when manufacturers choose older predicates
FDA is modernizing its 510(k) program and one change it is mulling is placing a limit on how old a predicate device is when manufacturers develop a new product showing substantial equivalence.