Abbott
-
Devices & Diagnostics, Health Tech
Abbott-Bigfoot Deal Proves That Diabetes Is a Hot Area for Digital Health Investment
This week, Abbott announced its plans to acquire Bigfoot Biomedical — a startup selling a “smart” insulin pen cap, which collects data from a user’s CGM to help them calculate the right dose. Most analysts were unsurprised to hear of the acquisition given that the two companies have been collaborating for more than six years, but they said the deal reinforces the steady pace of digital innovation in the diabetes care space.
-
Payer’s Place: Dawn Maroney
Dawn Maroney, President, Markets of Alignment Health and CEO of Alignment Health Plan, to discuss how they are using technology to provide better service and care to consumers.
-
Why Traditional Fertility Benefits Are Inequitable and Costing Employers More
Traditional fertility benefit practices — like dollar cap benefits and step therapy — are creating inequities in treatment, said Ann Gaines, senior vice president of business development at Progyny. Gaines made these comments Wednesday during the Midwest Business Group on Health conference in Chicago.
-
Devices & Diagnostics, Health Tech, SYN
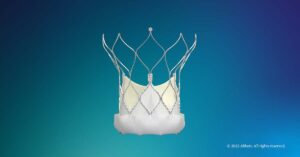
Abbott Enters TAVI Device Market Dominated by Edwards, Medtronic
Abbott recently earned FDA approval for its latest transcatheter aortic valve implantation (TAVI) system, which is named Navitor. Abbott will have to fight for its place the U.S. TAVI device space, as the systems made by Edwards and Medtronic have a steadfast command of the market share.
-
How medtech can crack the patient engagement conundrum and transform the patient experience
A look at how tapping real world evidence from medical devices and patients could be a game changer, improving patient-clinician communication, supporting earlier interventions and helping patients manage their condition.
-
The medtech industry’s new paradigm: Real world evidence collection
Innovative approaches to real world evidence collection present opportunities for medical device companies to improve patient outcomes. But success will depend on improving the way medtech companies integrate digital health. Executives from Abbott, Smith & Nephew, AliveCor and Huma weigh in on these issues.
-
Abbott’s HeartMate 3 extends life by 5 years for advanced heart failure patients
It is the first LVAD trial to examine five-year outcomes and surpasses a previous Abbott study on HeartMate 3, which recorded a survival time of two years. The device was found to reduce morbidity and mortality, largely because of fewer deaths by stroke, clotting and bleeding.
-
Project seeks to chart regulatory paths for digital products
The nonprofit Digital Medicine Society is partnering with the likes of Google and the FDA to develop a free resource designed to help digital health startups navigate the regulatory process.
-
Abbott is out to win hearts and minds. Will it one day become a household name?
Abbott wants to leap into the world of consumer wearables to track various health signals for better health for consumers and better growth of its business. Will it win hearts and minds?
-
Applying Remote Patient Monitoring to Surgery Prep and Recovery, Oncology and Women’s Health
Join us to learn about the latest trends in remote monitoring and how to extend its benefits beyond chronic conditions to more patients – all while using fewer staff resources.
-
Medical device companies should consider embracing connected care platforms
A recent webinar hosted by MedCity News and sponsored by BioT shared insights on some of the ingredients to successful creation and execution of remote patient monitoring solutions supported by a cloud-based connected care platform with speakers from BioT and Abbott.
-
FDA to review data on stroke-prevention implants after study finds more adverse events in women
A study published in August found that women experienced more adverse events from Boston Scientific’s Watchman implant than men. The FDA said it plans to take a closer look at data for these stroke-prevention implants, but still says the benefits outweigh the risks.
-
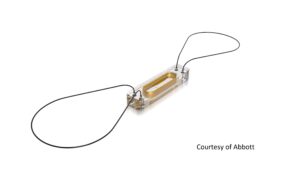
Abbott seeks expanded indication, national coverage for CardioMEMS device
With the results of a recent trial, Abbott is seeking an expanded indication from the FDA for its CardioMEMS device, intended to prevent hospitalizations in patients with heart failure. It’s also hoping to win over national coverage from CMS.
-
Abbott gets FDA clearance for CGM app
Abbott received FDA clearance for an app to pair with its Freestyle Libre 2 glucose monitor. The app lets people get readings directly on their phone without using a reader.
-
FDA approval brings remote programming for Abbott’s deep brain stimulation device
Abbott announced the launch of the newly-approved NeuroSphere Virtual Clinic by which deep brain stimulation patients can have their devices programmed and reset remotely, from the comfort of their homes without having to travel to a specialist.
-
As problems grow with Abbott’s fast Covid test, FDA standards are under fire
False negatives increase the risk that patients will not self-isolate or exercise other precautions — such as wearing a mask — and make more people sick than if they had had an accurate diagnosis.