Denmark
-
Pharma, Artificial Intelligence, BioPharma
Novo Nordisk Turns to Flagship’s Valo Health for AI-Driven Cardio Drug R&D
Novo Nordisk is paying Valo Health $60 million up front to gain three preclinical cardiovascular disease programs. Milestone payments for those programs and others covered under the artificial intelligence drug discovery pact could reach up to $2.7 billion.
-
Novo Nordisk’s Wegovy Posts Trial Data Supporting the Drug as Way to Lower Heart Risks
Novo Nordisk weight-loss drug Wegovy reduced the risk of major cardiovascular events by 20% in a large clinical trial. The company said the results support expanding the drug’s label, but the looming question is whether the data will persuade payers to cover the pricey chronic therapy.
-
Payer’s Place: Dawn Maroney
Dawn Maroney, President, Markets of Alignment Health and CEO of Alignment Health Plan, to discuss how they are using technology to provide better service and care to consumers.
-
Novo Nordisk Follows Eli Lilly’s Lead, Lowering Insulin Prices up to 75%
Novo Nordisk can afford the price cuts prices to several branded and non-branded insulins. Flattening revenue for the pharmaceutical giant’s insulin products is offset by demand for diabetes and obesity drugs from its semaglutide franchise, which continues to grow by leaps and bounds.
-
Blood Clotting Biotech Hemab Hauls In $135M to Drug Rare Bleeding Disorders
Hemab Therapeutics will apply its Series B financing toward clinical development of its lead drug candidate, a potential treatment for Glanzmann thrombasthenia. The capital will also support the rest its pipeline addressing rare bleeding and thrombotic diseases with no approved therapies.
-
Novo Nordisk’s Diabetes Pill Rybelsus Gets FDA O.K. as First-Line Therapy
The FDA approved a change in Rybelsus’s label, allowing the drug to be used as an initial therapy for type 2 diabetes patients. The once-daily pill has already become a blockbuster product for Novo Nordisk.
-
LEO Pharma’s FDA nod adds a challenger to Dupixent’s dominance in eczema
With FDA approval for its atopic dermatitis drug Adbry, LEO Pharma will reach the U.S market ahead of an Eli Lilly atopic dermatitis drug that addresses the same target. LEO will also able to offer patients an alternative to the blockbuster Sanofi/Regeneron Pharmaceuticals drug Dupixent.
-
Novo Nordisk reaches $3.3B deal to acquire RNAi partner Dicerna Pharma
The acquisition agreement comes exactly two years after the two companies began a wide-ranging alliance focused on developing RNA interference drug for metabolic disorders. The most advanced therapy from that alliance is on track to reach the clinic in 2022.
-
Seagen, Genmab antibody drug conjugate gets FDA approval in cervical cancer
The Seagen and Genmab antibody drug conjugate (ADC), named Tivdak, is approved to treat cases of cervical cancer that have returned or spread following treatment with chemotherapy. Tivdak is Seagen’s third ADC; for Genmab, the drug will become its first commercialized product.
-
Novo Nordisk cardio push picks up Phase 2-ready Prothena drug in $100M deal
Novo Nordisk is paying $100 million up front to acquire an experimental Prothena drug being developed to treat heart problems stemming from a misfolded protein. The deal is part of a broader strategy to expand into drugs for cardiovascular disease.
-
Applying Remote Patient Monitoring to Surgery Prep and Recovery, Oncology and Women’s Health
Join us to learn about the latest trends in remote monitoring and how to extend its benefits beyond chronic conditions to more patients – all while using fewer staff resources.
-
FDA rejection of rare disease drug leads Orphazyme to cut two thirds of staff
Orphazyme’s corporate restructuring comes less than two weeks after the FDA rejected arimoclomol, a drug developed to treat the rare Niemann-Pick disease type C. The company said it is now focused on securing regulatory approval in Europe and finding a path forward for the small molecule with the FDA.
-
Two new biotechs unveil $112M combined for antibody drug conjugates for cancer
The list of FDA-approved antibody drug conjugates (ADC) is growing, and two more biotech startups have emerged from stealth with new cash and new approaches to this type of cancer drug. Adcendo and Adcentrx raised a combined $112 from their Series A financings.
-
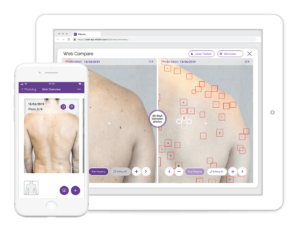
Skin-checking app sees future in clinicians, not automation
Instead of suggesting a diagnosis for moles and skin lesions, digital health startup Miiskin is leaving that up to dermatologists. The company is rolling out a version of its tool for clinicians to make it easier for their patients to track moles and other spots on their skin.
-
AbbVie, Denmark’s Genmab enter nearly $4B cancer immunotherapy partnership
The deal, which includes a hefty $750 million upfront payment to Genmab, focuses on bispecific antibodies and also a drug discovery research partnership.
-
FDA approves first monkeypox vaccine, which also protects against smallpox
The agency announced the approval of Bavarian Nordic’s Jynneos. A 2003 outbreak in the Midwest marked the first time monkeypox was found outside of its native Africa. Smallpox has been eradicated, but bioterrorism concerns remain.
-
FDA approves Novo Nordisk’s oral Type 2 diabetes drug
The drug, Rybelsus, is the first oral medication in the GLP-1 receptor agonist class. The approval was based on data from 10 trials that enrolled more than 9,500 patients.