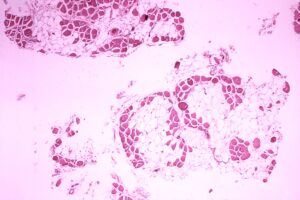
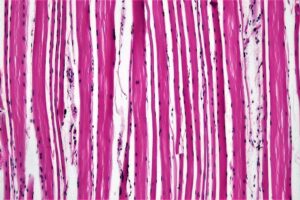
duchenne muscular dystrophy
-
MedCity Pivot Podcast: Tackling Rare Disease With Someone Who Was Touched By It
Rich Horgan believes that there’s an alternative path to drug development that can lower costs and the time it takes to bring drugs to market. He is trying to apply it to the field of rare and ultra-rare diseases.
-
FDA Approves First Gene Therapy for Duchenne Muscular Dystrophy
Sarepta Therapeutics’ Elevidys is now the first FDA-approved gene therapy for Duchenne muscular dystrophy. Elevidys is an engineered version of a gene intended to restore function lost to the mutation at the root of the inherited muscle disease.
-
Payer’s Place: Dawn Maroney
Dawn Maroney, President, Markets of Alignment Health and CEO of Alignment Health Plan, to discuss how they are using technology to provide better service and care to consumers.
-
Startup aiming to push boundaries of gene therapy nets $55M in seed cash
Private equity firm KKR teamed up with OMX Ventures to lead the seed investment in Replay, a company developing a suite of technologies that could overcome capacity limitations of adeno-associated viruses used for genetic medicines delivery. Duchenne muscular dystrophy is among the disease targets of the new startup.
-
With new gene therapy data in hand, Sarepta talks with FDA about approval pathways
Sarepta Therapeutics has more clinical data showing the safety and efficacy of its experimental gene therapy for Duchenne muscular dystrophy. A pivotal Phase 3 test is already underway and could post data next year but the company is also talking with regulators about the possibility of a submission under the accelerated approval pathway.
-
As muscular dystrophy drug meets key goal, Italfarmaco plans march forward to regulators
A Duchenne muscular dystrophy drug candidate from Italfarmaco Group has encouraging preliminary data from a pivotal study. The Milan, Italy-based pharmaceutical company says it now plans to meet with U.S. and European authorities about seeking regulatory approval for the small molecule.
-
Code Bio corrals $75M to skip viruses, use synthetic DNA for genetic meds delivery
Code Biotherapeutics uses synthetic DNA as the foundation for its genetic medicines, which the startup claims offer several key advantages compared to genetic medicines delivered via engineered viruses. The startup plans to use its new capital to develop lead programs in Duchenne muscular dystrophy and type 1 diabetes.
-
PepGen IPO nabs $108M for muscular dystrophy drug with potential edge over Sarepta med
PepGen’s technology improves the delivery of a therapy to more tissue types and the clinical-stage biotech plans to use its IPO cash to continue developing its lead program for Duchenne muscular dystrophy. Meanwhile, eye products giant Bausch + Lomb returned to the public markets as a standalone company. Both the PepGen and Bausch + Lomb IPOs priced below their respective price ranges.
-
FDA lifts Pfizer gene therapy clinical hold, but trial adds new safety measures
Pfizer now has the regulatory O.K. to resume pivotal tests of its gene therapy for Duchenne muscular dystrophy—but with new limitations and safety measures. The FDA had placed a clinical hold on the study late last year following a patient death.
-
Patient death prompts FDA halt on Pfizer’s Duchenne gene therapy study
The FDA has halted tests of a Pfizer gene therapy for Duchenne muscular dystrophy. The clinical hold is one of several placed on gene therapy tests in the past year due to safety concerns.
-
Applying Remote Patient Monitoring to Surgery Prep and Recovery, Oncology and Women’s Health
Join us to learn about the latest trends in remote monitoring and how to extend its benefits beyond chronic conditions to more patients – all while using fewer staff resources.
-
Capricor’s muscle drug data look good, but partner wanted and Phase 3 test needed
Capricor Therapeutics’ cell therapy for Duchenne muscular dystrophy posted encouraging results from a Phase 2 study enrolling 20 patients. But the FDA said a Phase 3 test is needed to support a regulatory submission, and the biotech is hunting for a pharmaceutical industry partner.
-
FDA approves Sarepta drug for muscular dystrophy with rare genetic mutation
The FDA decision gives Sarepta Therapeutics its third approved drug for Duchenne muscular dystrophy. The accelerated approval requires the biotech to conduct additional clinical testing to confirm the drug’s benefit.
-
Documents give inside view of FDA’s rejection, and surprise approval, of Sarepta Duchenne’s drug
Despite a strongly critical complete response letter – which the FDA sent in August but did not make public until Tuesday – the drugmaker successfully appealed and won approval for the drug, Vyondys 53, in December, to the surprise of many.
-
Roche, Sarepta Therapeutics sign $1.15B deal for ex-US rights to gene therapy for Duchenne’s
An analyst wrote that the deal would create value for Sarepta, which has a gene therapy in Phase II development for Duchenne muscular dystrophy, SRP-9001, but lacks the resources to commercialize it abroad.
-
Sarepta wins surprise approval for second Duchenne muscular dystrophy drug
The FDA had previously rejected Sarepta’s application for Vyondys 53 in August, citing infections and kidney toxicity in preclinical studies. However, some analysts saw the move as a ‘slap on the wrist’ for the controversial approval of Sarepta’s first DMD drug.
-
FDA rejection of Sarepta drug may be a ‘slap on the wrist’ over earlier approval, analyst writes
In a note to investors, SVB Leerink analyst Joseph Schwartz suggested that the ‘questionable’ approval of Sarepta’s Exondys 51 may have played a role in the FDA’s decision to turn down golodirsen.