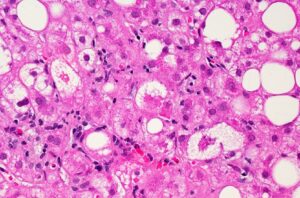
nonalcoholic steatohepatitis
-
After NASH Hopes Are Dashed, Intercept Pharma Agrees to $794M Buyout
Intercept Pharmaceuticals’ acquisition by Alfasigma comes three months after the FDA again rejected the biotech’s drug as a treatment for the fatty liver disease NASH. But Intercept still has rare liver disease assets, and Italy-based Alfasigma says acquiring the company will help it expand in gastroenterology and hepatology.
-
Two Biotechs Break the IPO Dry Spell, Raising $385M Combined
Inflammation and immunology biotech Apogee Therapeutics and Sagimet Biosciences, a company developing a drug for the fatty liver disease NASH, are the latest biotech companies to join the public markets. Both companies upsized their IPOs, raising cash for clinical trials.
-
Payer’s Place: Dawn Maroney
Dawn Maroney, President, Markets of Alignment Health and CEO of Alignment Health Plan, to discuss how they are using technology to provide better service and care to consumers.
-
Ipsen gets rights to failed Genfit NASH drug, now in Phase 3 for rare liver disease
Ipsen is paying €120 million up front to acquire global rights to elafibranor, a Genfit drug that fell short as a treatment for NASH, but is currently in Phase 3 testing in another liver disease, primary biliary cholangitis. Meanwhile, Genfit is adding an early-stage drug candidate to its own pipeline via a separate transaction.
-
GSK joins NASH chase, paying $120M up front for rights to Arrowhead RNAi drug
GlaxoSmithKline sees enough promise in the early clinical data of a novel NASH drug to pay its developer, Arrowhead Pharmaceuticals, $120 million up front for rights to the experimental therapy. The Arrowhead drug uses RNA interference to “silence” an enzyme associated with the progression of the fatty liver disease.
-
Gilead and Novo Nordisk advance NASH collaboration to larger clinical study
Clinical tests of drugs from Gilead Sciences and Novo Nordisk have yielded encouraging results in nonalcoholic steatohepatitis (NASH), and the partners now want to see if the drug combinations help patients in a larger mid-stage study. The planned Phase 2b study expands on a 2019 R&D alliance.
-
Merck, Hanmi sign $870M deal for NASH drug
Hanmi will retain options for the drug, efinopegdutide, in Korea under the deal, in which Merck is paying the Seoul-based company $10 million upfront and up to $860 for development, regulatory and commercialization milestones.
-
INVEST Pitch Perfect winner spotlight: Pleiogenix eyes road ahead for liver disease drug
The company is hoping to close its seed funding round in the next six months and aims to be in a position to seek regulatory approval for its lead asset, PLX888, in acute alcoholic hepatitis in 2023.
-
French biotech Genfit shifts gears after Phase III NASH failure
The company said it was formally terminating the Phase III study of the drug elafibranor in NASH and refocus its development of the drug on another disease, primary biliary cholangitis, as well as a diagnostic for NASH.
-
Applying Remote Patient Monitoring to Surgery Prep and Recovery, Oncology and Women’s Health
Join us to learn about the latest trends in remote monitoring and how to extend its benefits beyond chronic conditions to more patients – all while using fewer staff resources.
-
Glympse Bio raises more than $46M in Series B round for biosensor technology
The company is developing biosensors, initially for NASH, that could be used in place of liver biopsy and have applicability in development of drugs for the disease.
-
FDA turns down Intercept’s long-anticipated NASH drug
The FDA has requested additional data from the company’s clinical trial. Intercept’s CEO expressed disappointment at the decision, saying the agency had not communicated that the drug, obeticholic acid, was not approvable.
-
Intercept hits regulatory snag at FDA, likely delaying NASH drug’s approval
The company said that the agency had requested additional data for obeticholic acid and thus pushed the upcoming advisory committee meeting back from June 9, thereby delaying its decision on approval, which had also been scheduled for June 26.
-
Genfit’s drug flops in Phase III, joining graveyard of NASH failures
The French drugmaker said Monday that its Phase III study of elafibranor failed to meet its primary and key secondary endpoints. Analysts remarked that this extends the lead of the drug likely to get the first NASH approval, Intercept’s obeticholic acid.
-
The top biopharma companies targeting NASH
These are four companies that currently have drugs in Phase III development for nonalcoholic steatohepatitis or have them under Food and Drug Administration review.
-
Boehringer Ingelheim dumps NASH drug program amid concerns over drug interactions
BI said it would continue to develop the drug, BI 1467335, in other diseases and remain committed to NASH overall. Shares of the Australian company from which it licensed the drug, Pharmaxis, fell more than 40%.