liver disease
-
After NASH Hopes Are Dashed, Intercept Pharma Agrees to $794M Buyout
Intercept Pharmaceuticals’ acquisition by Alfasigma comes three months after the FDA again rejected the biotech’s drug as a treatment for the fatty liver disease NASH. But Intercept still has rare liver disease assets, and Italy-based Alfasigma says acquiring the company will help it expand in gastroenterology and hepatology.
-
After Second FDA Rejection, Intercept Abandons NASH Drug and Restructures
The FDA again rejected Intercept Pharmaceuticals’ application seeking accelerated approval for its NASH drug and asked for more data. Instead, the biotech will stop all work in that fatty liver disease and focus on drugs for other serious but rare liver conditions.
-
Payer’s Place: Dawn Maroney
Dawn Maroney, President, Markets of Alignment Health and CEO of Alignment Health Plan, to discuss how they are using technology to provide better service and care to consumers.
-
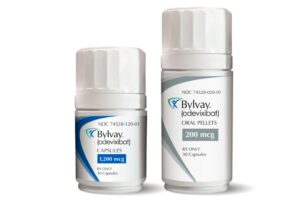
Ipsen Itching Drug Scratches Off a Second FDA Approval in Rare Liver Indication
Ipsen drug Bylvay is now FDA approved for treating pruritus, or severe itching, which is a complication of the rare liver disease Alagille syndrome. The oral drug was previously approved for treating pruritus in another rare inherited liver disease called PFIC.
-
NASH Drug Biotech 89bio Nails Phase 2 Goals; Next Up Is a Pivotal Clinical Trial
89bio reported its NASH drug candidate met the main endpoints of a mid-stage clinical trial. In addition to demonstrating efficacy and safety according to measures the FDA says are needed to support a regulatory submission, 89bio says the results also show its drug could offer a dosing edge over potential rivals.
-
Artificial Intelligence, BioPharma
RNA therapies startup Ochre Bio lands $30M to take on chronic liver disease
Ochre Bio’s Series A financing will support development of RNA therapies for chronic liver disease. Longer term, the company aims to develop therapies that regenerate organs in patients, potentially avoiding the need for organ transplants.
-
Genfit spreads its bets in a rare liver disease with buyout of Phase 2-ready biotech
Liver disease remains the focus of Genfit, despite a high-profile clinical trial failure in the fatty liver disease NASH. The company is building out its pipeline by acquiring Versantis, a clinical-stage biotech whose most advanced drug candidate brings a novel approach to treating a deadly chronic liver disease complication that has no FDA-approved therapies.
-
After initial failure, Intercept Pharma feels new data can get NASH drug approval
Two years after the stinging FDA rejection of its drug for the fatty liver disease NASH, Intercept Pharmaceuticals has more safety and efficacy data from a pivotal study that could support resubmission of a new drug application. The biotech said it will meet with the FDA later this month.
-
Regenerative med biotech Satellite unveils tissue-based tech to restore organ function
Satellite Bio has emerged from stealth with technology for bioengineering tissue to restore organ function. The regenerative medicine startup, based on research from MIT and Boston University, is backed by $110 million in financing.
-
Ipsen gets rights to failed Genfit NASH drug, now in Phase 3 for rare liver disease
Ipsen is paying €120 million up front to acquire global rights to elafibranor, a Genfit drug that fell short as a treatment for NASH, but is currently in Phase 3 testing in another liver disease, primary biliary cholangitis. Meanwhile, Genfit is adding an early-stage drug candidate to its own pipeline via a separate transaction.
-
Applying Remote Patient Monitoring to Surgery Prep and Recovery, Oncology and Women’s Health
Join us to learn about the latest trends in remote monitoring and how to extend its benefits beyond chronic conditions to more patients – all while using fewer staff resources.
-
Atlas Venture-incubated Rectify lands $100M to transport drug hunt to new grounds
Rectify Pharmaceuticals is taking an approach to disease that led to a successful franchise of Vertex Pharmaceutical lung drugs and expanding it to more diseases in more organs in the body. The startup has launched with $100 million in financing.
-
Geisinger’s latest work: An upcoming patient transportation initiative and new insight on chronic liver disease
The Danville, Pennsylvania-based system plans to work with rabbittransit to help patients get to their appointments on time. Additionally, researchers from Geisinger and Regeneron Genetics Center recently found a gene variant that’s associated with a reduction in the risk for chronic liver disease.
-
With $100 million buy, BMS targets “richest opportunity for blockbuster drugs”
Gilead just lost one candidate, Bristol-Myers has licensed another; the race for non-alcoholic fatty liver disease drugs continues, as many companies eye its potential for a blockbuster drug.
-
Devices & Diagnostics, Startups
Swiss maker of pump for late stage liver disease complications raises $9.3M
Sequana Medical wants to embark on clinical trials in the U.S. for its implantable pump to treat complications from cirrhosis.
-
Forget mood rings – check out the color of your pee to see what’s going on
The color of your urine could be telling you something about your health condition. Yes, […]