carlsbad
-
Novartis Pays Ionis $60M to Expand Alliance on a Hot Target for Cardio Drugs
Novartis and Ionis Pharmaceuticals, already partners in cardiovascular disease R&D, are expanding their alliance to see if they can develop a next-generation therapy offering efficacy and dosing advantages. But competitors are already in the mix with programs addressing the same cardiovascular disease target.
-
Payer’s Place: Dawn Maroney
Dawn Maroney, President, Markets of Alignment Health and CEO of Alignment Health Plan, to discuss how they are using technology to provide better service and care to consumers.
-
AstraZeneca drops Ionis-partnered heart drug after Phase 2 success isn’t good enough
An Ionis Pharmaceuticals heart disease drug beat a placebo in a mid-stage clinical trial, but partner AstraZeneca has decided it won’t advance the genetic medicine to a larger Phase 3 test. The results were apparently not enough to set the Ionis therapy apart from commercialized cholesterol-lowering drugs that address the same protein target.
-
Roche pays $55M for late-stage drug for rare kidney disease with no approved treatment
An Ionis Pharmaceuticals drug has positive Phase 2 results in immunoglobulin A nephropathy, leading Roche to exercise its option to license the rights to the molecule. The pharmaceutical giant gets a contender in the chase to win the first regulatory approval of a therapy for this rare kidney disease.
-
Aiming to catch Alnylam, AstraZeneca & Ionis plan FDA filing for rare disease drug
The planned FDA submission follows the report from AstraZeneca and Ionis Pharmaceuticals that their partnered drug, eplontersen, met the main goals of a pivotal study in treating nerve pain caused by hereditary transthyretin-mediated amyloidosis. The data come one week after rival Alnylam Pharmaceuticals won FDA approval for its second drug for this rare disease.
-
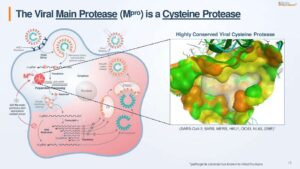
Not yet in the clinic, Pardes Bio’s Covid-19 pill still fills in Foresite’s blank check
Pardes Biosciences advanced from concept to drug candidate in less than nine months. The preclinical biotech is developing an oral antiviral for Covid-19 and other coronavirus infections and it is going public in a SPAC merger that will infuse it with $276 million.
-
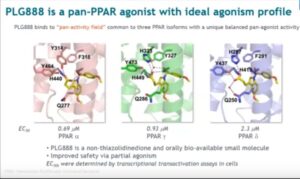
INVEST Pitch Perfect winner spotlight: Pleiogenix eyes road ahead for liver disease drug
The company is hoping to close its seed funding round in the next six months and aims to be in a position to seek regulatory approval for its lead asset, PLX888, in acute alcoholic hepatitis in 2023.
-
Dynacure secures $55 million Series A round for rare genetic disease treatment
French firm to start trial of Dyn101, in-licensed from US-based Ionis Pharmaceuticals, in centronuclear myopathy next year.
-
Applying Remote Patient Monitoring to Surgery Prep and Recovery, Oncology and Women’s Health
Join us to learn about the latest trends in remote monitoring and how to extend its benefits beyond chronic conditions to more patients – all while using fewer staff resources.
-
Here’s the latest entrant in the Internet of Things in healthcare market
As virtual rehabilitation picks up, traditional orthopedics widget makers like Breg are foraying into at-home physical therapy using a sensor device and a companion mobile app.
-
Devices & Diagnostics, Startups
Will a device approach to obesity work ? IPO-seeker Obalon hopes so
Obalon has developed a gastric balloon system to help overweight patients shed extra pounds, but will the device work over the long term?