New York
-
Brainstorm’s ALS Data Fail to Persuade FDA Advisors, Who Vote Down the Stem Cell Therapy
NurOwn, Brainstorm Cell Therapeutics’ experimental stem cell therapy for ALS, did not win the backing of an independent panel of FDA advisors. Many advisory committee members said they want to see more data from another clinical trial, the same guidance the FDA has given the biotech for nearly three years.
-
After NASH Hopes Are Dashed, Intercept Pharma Agrees to $794M Buyout
Intercept Pharmaceuticals’ acquisition by Alfasigma comes three months after the FDA again rejected the biotech’s drug as a treatment for the fatty liver disease NASH. But Intercept still has rare liver disease assets, and Italy-based Alfasigma says acquiring the company will help it expand in gastroenterology and hepatology.
-
Payer’s Place: Dawn Maroney
Dawn Maroney, President, Markets of Alignment Health and CEO of Alignment Health Plan, to discuss how they are using technology to provide better service and care to consumers.
-
Devices & Diagnostics, BioPharma
Click Expands to Substance Use Disorder With Indivior Digital Therapeutics Pact
Digital therapeutics developer Click Therapeutics is partnering with Indivior to develop a mobile app for substance use disorder. Indivior is now Click’s third development partner, following deals with Boehringer Ingelheim and Otsuka Pharmaceutical.
-
GSK Patent Suit Aims to Halt Pfizer’s RSV Vaccine for Adults, But Not Infants
GSK claims that Pfizer’s FDA-approved vaccine for respiratory syncytial virus infringes on four patents protecting its own approved vaccine, Arexvy. The patent suit comes with the RSV season approaching.
-
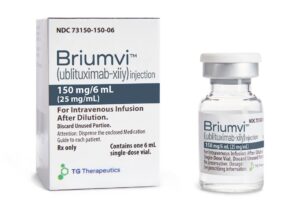
TG Therapeutics Lands Partner to Market MS Drug in Europe and Beyond
TG Therapeutics’ multiple sclerosis drug Briumvi will be commercialized in Europe by Neuraxpharm. The Germany-based company paid $140 million up front for certain rights to the drug, which received European Commission approval in June.
-
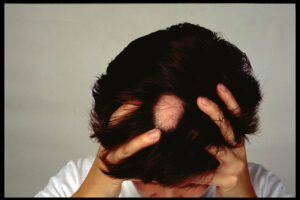
FDA Approval of Pfizer Alopecia Drug Brings New Competition to Eli Lilly Med
Pfizer’s Litfulo is the first treatment approved for treating adolescents with severe alopecia areata. The drug’s approval also covers adults, were it will compete against Eli Lilly’s Olumiant.
-
Pfizer Hemophilia Drug Reduces Bleeding in Key Study; Regulatory Filings Are Planned
Marstacimab, a Pfizer drug for both hemophilia A and B, met the main goal of a Phase 3 study and the company now plans to discuss the data with regulators. The Pfizer drug could beat to the market a Novo Nordisk drug that addresses the same novel target.
-
Devices & Diagnostics, BioPharma
Labcorp Shells Out $146M to Buy Enzo Biochem’s Clinical Lab Business
Enzo BioChem’s sale of its clinical lab business to Labcorp is the culmination of a monthslong strategic review. Meanwhile, diagnostics and lab testing giant Labcorp is in the middle of its own corporate shakeup as it readies its clinical trials business for a spinoff as a standalone, publicly traded company.
-
Pfizer’s Migraine Drug Approval Triggers $475M Payment to Royalty Pharma
Royalty Pharma’s milestone payment from the approval of Pfizer migraine drug Zavzpret stems from financing deals struck with the therapy’s previous developer, Biohaven Pharmaceuticals. Royalty Pharma is also in line to receive royalty payments going beyond the next decade.
-
Applying Remote Patient Monitoring to Surgery Prep and Recovery, Oncology and Women’s Health
Join us to learn about the latest trends in remote monitoring and how to extend its benefits beyond chronic conditions to more patients – all while using fewer staff resources.
-
Pfizer’s Seagen Acquisition Brings It Full Circle in a Hot Area of Cancer Drug R&D
Pfizer is no stranger to antibody drug conjugate cancer therapies, but it hasn’t been commercially successful with them. The pharma giant’s agreement to acquire Seagen for $43 billion will thrust it to the forefront of the ADC drug class, which has become a hot space for research and regulatory activity in recent years.
-
Where Big Pharmas Faltered, Stemline Succeeds and Lands FDA Nod in Breast Cancer
Orserdu, a drug from Menarini Group subsidiary Stemline Therapeutics, is now approved for treating breast cancers that carry the ESR1 mutation. The drug is the first approved oral therapy from a class of therapies called selective estrogen receptor degraders (SERDs).
-
FDA Again Spurns BrainStorm’s ALS Data, This Time With Official Refusal
BrainStorm Cell Therapeutics’ stem cell therapy for amyotrophic lateral sclerosis received an FDA refuse-to-file letter, correspondence that informs a company its application seeking approval is incomplete. The notice means BrainStorm might need to run another clinical trial.
-
Artificial Intelligence, BioPharma
Sanofi Inks Another AI Alliance, This Time Partnering With Insilico Medicine
Sanofi will use Insilico Medicine’s tech to advance drug development candidates for up to six new targets. Disease indications were not disclosed, but the pharmaceutical giant said the new agreement will boost its drug discovery research in China.
-
Pfizer RSV Vaccine Hits Trial Goal for Infants; Year-End FDA Filing Planned
Pfizer’s respiratory syncytial virus vaccine candidate infants has clinical data showing it helped prevent severe infections in infants. It’s a maternal vaccine that produces antibodies in the mother that confer temporary protection to a baby.
-
Artificial Intelligence, BioPharma
Sanofi exec jumps to Owkin to ramp up the AI biotech’s pharma partnership plans
Alban de La Sablière, global head of partnering at Sanofi for the past six years, has joined artificial intelligence biotech Owkin as its first chief business officer. In addition to finding more business partnerships, de La Sablière will also oversee expansion of Owkin’s internal drug and diagnostics pipeline.